
The ongoing, two-part Phase I study is assessing the safety and tolerability of MRx0518 monotherapy in treatment-naïve subjects undergoing surgical resection of solid tumors. MRx0518 in Neoadjuvant Setting Monotherapy We look forward to advancing our MRx0518 program further into the clinic and we are already enrolling patients into Part B of our Keytruda combination study, including the addition of new tumor type cohorts.”

The data generated from these trials provide us with huge confidence not only with moving forward with MRx0518 as a novel immunotherapy for the treatment of cancer, but also our MicroRx platform. “The data also furthers and clarifies our earlier work on the mechanism of action of MRx0518 and, importantly, the activity we are seeing in these patients mirrors the preclinical results, further validating our approach and the MicroRx platform.
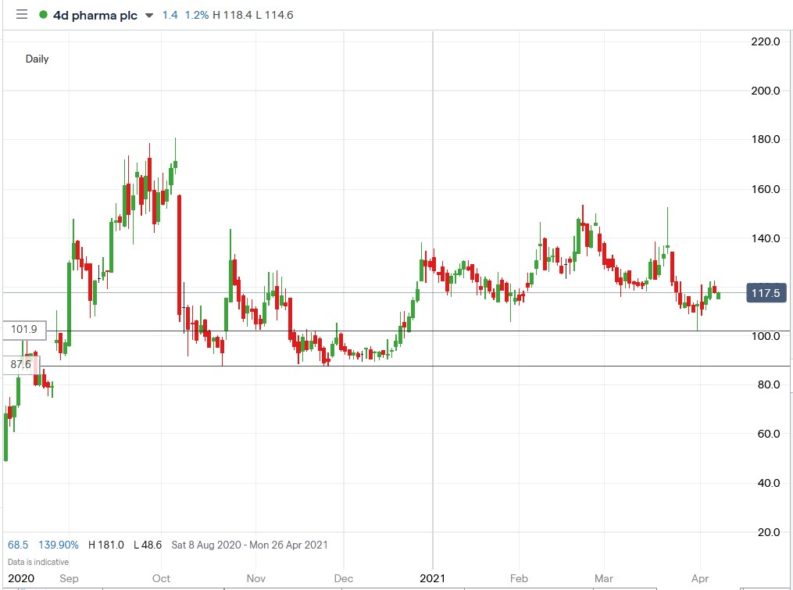
4d pharma oncology trial#
The strong immunological signals of biological activity shown in the monotherapy trial provides further clinical evidence of the role and contribution of MRx0518 to the impressive results we are seeing in combination with Keytruda in an incredibly difficult to treat patient population,” said Duncan Peyton, Chief Executive Officer of 4D pharma. “The data generated from both our trials of MRx0518 and presented this week at SITC, in monotherapy and combination with an immune checkpoint inhibitor settings, is further evidence of the potential of 4D’s LBPs in oncology.


 0 kommentar(er)
0 kommentar(er)
